Narcan® (naloxone hydrochloride) Nasal Spray is a brand name for an opioid antagonist used for emergency medical care of victims of opioid overdose. It offsets respiratory or mental depression caused by the use of opioids.
Naloxone is also an antidote for overdose of clonidine, a blood pressure medication.
https://www.narcan.com/home#how-to-use-narcan
How Does Naloxone Work on Opioid Receptors?
Naloxone blocks the effects of opioids–such as heroin, fentanyl, hydrocodone, or oxycodone–by attaching to the opioid receptors and reversing the opioid’s depression of the central nervous and respiratory systems.
Why is an Opioid Antagonist Needed?
Abuse of opioids continues to be a major U.S. public health issue. More than 101,750 reported fatal overdoses occurred during 2022, primarily driven by synthetic opioids such as fentanyl.
An easy-to-use opioid antagonist like Narcan blocks the physiological effect of the opioids and can save thousands of lives that might otherwise be lost.
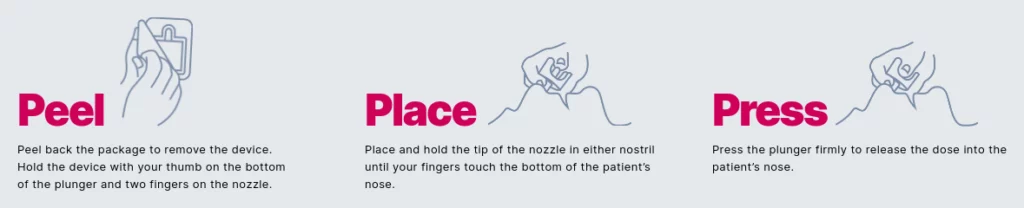
How to Use Naloxone Nasal Spray
If an opioid overdose is suspected, naloxone nasal spray should be injected into their nostril and the subject should then be rolled onto their side. Call 911 and watch the subject continuously while waiting for emergency medical care to arrive.
If there is no improvement after two to three minutes, a second dose should be administered. Should the situation require multiple doses, the nasal spray comes with two doses per package. Naloxone does not have an overdose potential and there are no adverse effects from multiple doses.
If the subject is not breathing or their breathing is very weak, begin cardiopulmonary resuscitation (CPR) if you know the technique.
In most instances, the nasal spray rapidly reverses the opioid overdose and the user returns to normal breathing. The effect of naloxone wears off after 30 to 90 minutes, so it is important to call emergency services.
Who Should Carry Narcan Nasal Spray?
Those living around people who have or are suspect of having an opioid addiction should carry naloxone as a precaution from the life-threatening possibility of an overdose.
A person overdosing on opioids is unable to self-administer naloxone. If they have naloxone, they should be sure that a family member or other person around them knows where they keep it.
Some pharmacies participate in government-sponsored programs that give out free Narcan or another OTC product containing generic naloxone. Check with your local pharmacy.
As with any medication, read the dosage instructions for inhaled or injectable naloxone and do not use them past their expiration date.
What are the Side Effects of Naloxone?
Side effects from naloxone are rare, but some people may be allergic to the medication.
People with opioid use disorder may have withdrawal symptoms within minutes after they are given naloxone. These may include headaches, increased blood pressure, rapid heart rate, sweating, runny nose, nausea, vomiting, abdominal cramps, pulmonary edema, body aches, and tremors.
The risk of death from an opioid overdose is a far worse outcome than severe opioid withdrawal symptoms.
How is Naloxone Taken?
To reverse overdoses, the primary methods to administer Naloxone are with a nasal spray or by injection with a hypodermic needle into muscle, under skin, or into a vein.
In a hospital, naloxone may be administered intravenously.

What are the Signs of Opioid Overdose?
The Substance Abuse and Human Services Administration (SAMHSA) says a person may be having an opioid overdose if:
- Their face is extremely pale and/or feels clammy to the touch
- Their body goes limp
- Their fingernails or lips have a purple or blue color
- They start vomiting or making gurgling noises
- They cannot be awakened or are unable to speak
- Their breathing or heartbeat slows or stops
Naloxone Hydrochloride Nasal Spray Development
Naloxone was patented in 1961 and prescription naloxone was approved for opioid use disorder treatment in the United States in 1971.
A Partnership Produced the Nasal Spray Version
A partnership of LightLake Therapeutics and the National Institute on Drug Abuse (NIDA) developed Narcan® Nasal Spray to treat victims of opioid overdoses.
The Federal Drug Administration (FDA) fast-tracked approval of its use as a prescription drug in 2015, the first FDA-approved nasal spray for emergency treatment of suspected opioid overdose.
In March 2023, the FDA approved Narcan for over-the-counter (OTC), nonprescription, use, making it the first naloxone product approved for use without a prescription.
First Aid for Opioid Overdose
Opioid overdose prevention kits were distributed by many states starting in 1996. The fact that medically untrained people can administer the simple nasal spray apparatus has made them a big success in preventing drug overdose deaths.
The Center for Disease Control (CDC) estimates that more than 26,000 cases of opioid overdose were reversed from 1996 to 2014 using the life-saving medication.
Where Can I Get Naloxone?
Many pharmacies carry naloxone. Some states, including Texas, allow purchase without a doctor’s prescription.
Some community-based distribution programs, local public health organization, or health departments provide it free of charge.
Co-Prescribing Naloxone
The U.S. Centers for Disease Control and Prevention recommends co-prescription of naloxone for some patients who take opioids. Research shows that also prescribing naloxone to persons taking prescription opioids may reduce the risk of prescription opioid overdose deaths.
Is Narcan® the same as naloxone?
Yes. There are many other formulations and brand names for naloxone, but the brand name Narcan® is often used for them instead of the proper generic name naloxone.
Sources:




